Complex Formulation
Complex Formulation
In terms of complex formulation, we own a sophisticated drug delivery technology platform which is capable of designing formulation that accurately matches the characteristics of each compound and indication. In particular, we have cutting-edge technologies on the sustained-release microsphere platform and inhalation formulation platform, allowing us to overcome various challenges in the development and manufacturing process.
Inhalation formulation platform
Inhalation formulations are complex formulations defined by the FDA, with high R&D and approval barriers. However, only a few pharmaceutical companies compete in this field due to the high entry barrier, suggesting significant market potential. Many inhalation products are included in China’s Catalog of Encouraged Generic Drugs, which includes drugs that are considered to be in short supply in China.
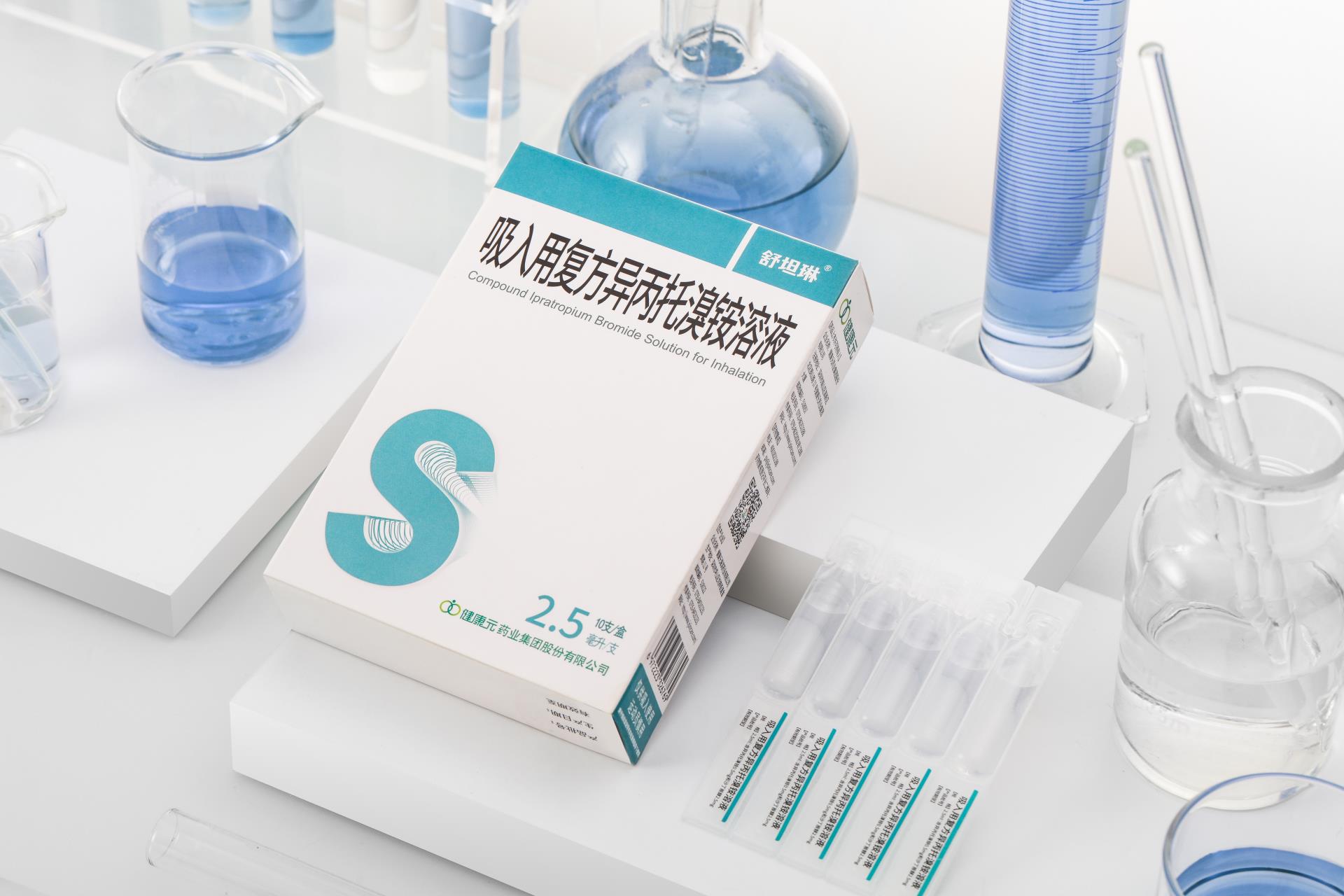
We have focused on inhalation formulations since 2013 and are one of the leading Chinese companies in this field. We have a comprehensive portfolio in the field of respiratory inhalation formulation, with rich drug products and drug candidates in the pipeline, as well as advanced R&D progress. Our landmark product, Shutanlin®, is the first Chinese inhalation product approved under the new registration classification issued by the NMPA.
As at 30 June 2022, we offered a number of inhalation products in China, including five commercialized products and nine specifications, one of which Budesonide Suspension Inhalation (0.5mg) was manufactured only by us among all Chinese companies. As at the same date, our drug candidates in the pipeline covered all forms of inhalation formulations, and 82 patent and patent applications in the field, of which 54 had been granted.
We are equipped with global-leading R&D capabilities, as evidenced by all formulation forms, effective drug-device linkage and high productivity, which enable us to provide more affordable medicines for patients in China. Leveraging our inhalation formulation platform, we seek to further develop our inhalation formulation to satisfy the unmet clinical needs for the treatment of respiratory diseases including idiopathic pulmonary fibrosis and lung infections. Particularly, we have accumulated extensive R&D experience in dry powder inhaler, aerosol, nebulised inhalation solution and other inhalation formulation.
Microsphere platform
Sustained-release microsphere for injection is a key innovation and R&D focus globally in the area of sustained-release formulation with significant market potential. Sustained-release microspheres can release packaged drug substances in a patient’s bloodstream over a period from a few weeks to a few months, which offers advantages of (i) reducing dosing frequency and improving patient compliance; (ii) improving bioavailability and stability by providing prolonged or controlled drug delivery, and (iii) being capable of targeting drug to specific sites. Microsphere injection production involves high technological barriers as encapsulation efficiency, particle size and distribution are difficult to control, making commercial production challenging.
we are one of the first Chinese companies to commercialize a sustained-release microsphere for injection products in China.
As at 30 June 2022, we were among only three Chinese pharmaceutical companies that successfully developed and commercialized sustained-release microsphere products.
Our sustained-release microsphere for injection technology platform fully encompasses the R&D and mass production technology system of long-term injection. As a national-grade engineering and research center in China, our sustained-release microsphere technology platform enabled us to build a rich product pipeline around sustained-release microspheres.

Quick Navigation
Service Hotline
Quick
Navigation
Service
Hotline

Official WeChat Official Account
Copyright © Joincare Pharmaceutical Group Industry Co., Ltd. All Rights Reserved. Powered by:www.300.cn Zhuhai 京ICP证000000号





